Post Operative Information

Introduction
Perioperative care is an important part of surgery and through much of this period the patient is jointly cared for by the anaesthetic and surgical teams. A clear understanding of the key elements of perioperative anaesthetic care is essential for the surgical trainee.
Postoperative monitoring needs to be appropriate to the needs of the individual patient and an understanding of both the indications and the technical aspects of the various monitoring modalities is necessary. Effective monitoring will lead to recognition of impending problems, allow preventive treatment and thus improve outcome.
While ventilatory support is administered by anaesthetists and intensive care teams, an understanding of the indications, ventilatory options and complications is essential to aid the surgeon in:
- caring for patients undergoing ventilatory support
- communicating effectively with our anaesthetic colleagues
Pain control is a key component of surgical care and has evolved and improved considerably over the years, with increased options in terms of both analgesic drugs and means of delivery. As perioperative care generally involves intravenous drug delivery, an understanding of the principles of IV drug delivery and the commonly used IV drugs in surgical practice is important.
Ventilatory Support
Although most patients recover uneventfully after an operation, a minority develop significant respiratory compromise. Because of the serious, or potentially fatal, consequences of respiratory failure, respiratory function must be closely monitored in at-risk patients and early intervention instituted where necessary. Such support can range from simply providing oxygen to endotracheal intubation for mechanical ventilatory support.
Types of Ventilatory Support
A variety of options are available for ventilatory support depending on the clinical scenario. Support can be negative pressure or positive pressure and invasive or noninvasive.
| Type of Support | Options |
|---|---|
| 1. Negative pressure: Non-invasive |
|
| 2. Negative pressure invasive: Endotracheal tube |
|
| 3. Positive pressure: Noninvasive |
|
| 4. Positive pressure invasive: Endotracheal tube |
|
Signs of Impending Respiratory Distress
Respiratory distress is generally readily recognisable to the trained eye. It consists of a constellation of physical signs including:
- tachypnea
- tachycardia
- hypertension
- sweating
- use of accessory muscles of respiration
- pulsus paradoxus
- speaking in short sentences
Ventilatory fatigue supercedes respiratory distress and precedes respiratory arrest. Likewise, it is a combination of physical signs comprising:
- cyanosis
- confusion or agitation followed by obtundation (due to hypoxic or hypercapnic narcosis)
Respiratory failure is a clinical condition characterised by abnormal arterial blood gases. While respiratory distress, ventilatory fatigue and respiratory arrest are clinical diagnoses, the diagnosis of respiratory failure is a biochemical one.
Objectives of Mechanical Ventilation
The basic objective of mechanical ventilation is to increase arterial pO2 and to decrease pCO2 levels. These objectives include:
- oxygenation of tissues using high FiO>2, PEEP, and ventilatory strategies in patients with severe respiratory failure due for instance to ARDS or flail chest.
- ventilation — gas exchange using ventilatory modalities in cases of severe respiratory failure including acute severe asthmatic attack and bronchopleural fistula.
- relief of respiratory distress — simple endotracheal intubation and ventilatory support without sedation may be sufficient to relieve distress. Conversely, agitation may interfere with gas exchange necessitating sedation and even neuromuscular blockade.
- prevention of secondary injury
- traumatic brain injury — hypoxaemia and hypercapnia both have deleterious effects on neurological outcome following traumatic brain injury. Endotracheal intubation and mechanical ventilation are indicated in a patient who has a Glasgow Coma Score of less than eight.
- acute myocardial infarction mechanical ventilatory support relieves respiratory distress associated with pulmonary oedema and assists left ventricular function with consequent improvement of haemodynamic profile. The application of continuous positive airway pressure (CPAP) via invasive or noninvasive means has the effect of increasing intrathoracic pressure and augmenting ventricular contraction, and reducing myocardial oxygen consumption.
- prevention of complications associated with mechanical ventilation such as barotrauma, volutrauma, Auto-PEEP, pneumonia, atelectasis, lobar/lung collapse and tracheal mucosal injury.
Methods of Artificial Mechanical Ventilation
Intermittent Positive Pressure Ventilation (IPPV)
IPPV is the most commonly used method of artificial ventilation for surgical anaesthesia and in the intensive care setting. IPPV is established with endotracheal intubation and muscle relaxation. The physiological effects of IPPV include:
- increased intrapleural pressure
- reduced lung compliance
- increased physiological dead space
- respiratory alkalosis
- diminished venous return
- diminished cardiac output
Continuous Positive Airways Pressure (CPAP):
In CPAP, positive pressure is applied to the airway of the patient who is breathing spontaneously. It is useful following cardiac surgery, in weaning patients from IPPV and also in respiratory distress of the newborn.
Positive End Expiratory Pressure (PEEP)
PEEP can be helpful especially when the arterial oxygen tension is low in spite of high FiO2, and in respiratory distress in the newborn. Special care must be taken in applying PEEP in hypovolaemia because PEEP further compromises venous return, in bronchospasm where expiration may already be impeded, and in the setting of rib fractures where it may predispose to pneumothorax. Harmful effects include diminished venous return and cardiac output and increased airways pressure that can result in barotrauma.
Intermittent Mandatory Ventilation (IMV)
The patient is allowed to breathe spontaneously but ventilation is augmented according to a preset minute volume. IMV is advocated during weaning from mechanical ventilation.
Pain Control
Postoperative pain control, though an essential part of surgical management and essential for good recovery, is often badly managed. Over 80% of patients experience pain of moderate or severe intensity during their postoperative course, 30% say that pain is present all or most of the time, 50% regard pain as being worse than expected and over 40% of patients have to request pain relief.
Psychological support is useful and can be given at the preoperative anaesthetic visit by the anaesthetist. Educating the patient about the perioperative period and pain relief can allay anxieties.
We will examine different analgesics and means of delivery:
Opioid Analgesics
Opioid analgesics are used to relieve moderate to severe postoperative pain. As a group, opioid analgesics share many side effects, though differences exist between different drugs. Common side effects of opioid analgesics include:
- nausea
- vomiting
- constipation
- drowsiness
- pruritus
- urinary retention
- respiratory depression
- hypotension
The key to using opioids is to titrate the dose against the desired effect so as to achieve optimal pain relief with minimal side effects. It is advisable to have a policy of one opioid in a surgical unit, thus allowing familiarity within the unit with dosage effects and problems.
Morphine is the gold standard and the first line opioid analgesic for severe acute pain. Side-effects may be more apparent during the accumulation of the active metabolite morphine-6-glucuronide in renal failure.
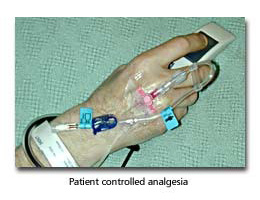
Patient Controlled Analgesia (PCA)
Traditional opioid delivery in the postoperative patient involves intermittent intramuscular or intravenous injections either as required or on a regular basis. Regular administration does not match patient need well and administration on an “as required” basis can be difficult to effectively deliver for practical reasons. Patient controlled analgesia is now very commonly used, and hands pain control back to the patient — providing effective analgesia matched to patient need and saving nursing time.
Essentially intravenous morphine delivery is through a pump connected to a readily accessible patient button. Analgesia is delivered when the patient presses the button and the pump controls the maximum allowable analgesic dosage and can also be set to deliver a regular baseline dosage. Patients can readily increase their analgesia for mobilisation or physiotherapy and decrease their dosage while resting in bed.
It has been shown that PCA combines more effective analgesia with smaller total analgesic dosages. Thorough monitoring protocols for sedation and respiratory depression are essential. All high-risk patients (those undergoing major surgery, the elderly, those with cardiovascular disease) on PCA analgesia should be given oxygen through nasal cannulas. When gastrointestinal tract function recovers PCA analgesia should be discontinued and the patient switched to oral analgesia, either opioid or non-opioid as appropriate.
Non-opioid Analgesics
Whereas opioid analgesics tend to be suitable especially for severe visceral pain, non-opioid analgesics are particularly suitable for musculoskeletal pain. Paracetamol is an effective analgesic for mild to moderate pain. It has no demonstrable anti-inflammatory activity. It causes significantly less gastric irritation than aspirin or NSAIDs and for this reason is the preferred drug for mild to moderate pain.
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) are effective in the relief of postoperative pain and where there are no clear contraindications, oral NSAIDs should be included in all postoperative pain relief plans. In renal colic they act most quickly when given intravenously, but for other conditions it seems that the route of administration is not important.
Side effects include constipation, renal and coagulation dysfunction and, in the long term, gastric bleeding. Acute renal failure can be precipitated in patients with pre-existing renal and cardiac impairment, those on loop diuretics, or those who have lost 10% or more of their blood volume. Short-term perioperative prescription of NSAIDs is contraindicated in poorly controlled asthma but in stable asthma should not cause clinical problems.
The commonly used NSAIDs are ibuprofen and diclofenac.
Regional Anaesthesia and Postoperative Rehabilitation
Regional anaesthesia can deliver optimal pain relief whilst minimising the systemic side effects of analgesic agents. Nerve blocks can provide good pain relief in the initial postoperative period, and there is no evidence to suggest that these patients then experience rebound pain after they have been discontinued.
Epidural Analgesia
Epidural infusions provide continuous pain relief following surgery on the lower limb, spine, abdomen or thorax. There is evidence that this pain relief results in a reduced risk of thromboembolism and respiratory complications. The risks associated with epidural use include:
- dural puncture
- infection
- haematoma
- nerve damage
- hypotension
- excessive motor block
- toxicity
- complications of opioids
The risk of persistent neurological damage after an epidural is 1 in 5000 and it is best to perform catheter insertion when the patient is awake before induction of anaesthesia. The coagulation profile of the patient must be confirmed as normal at insertion and at removal of the catheter, as an epidural haematoma, though rare, is a catastrophic event.
Heparin prophylaxis needs to be withheld on the morning of surgery and carefully timed with catheter removal. When opioids are administered via the epidural route, pain relief is produced as the drug diffuses slowly into the subarachnoid space and then passes to opioid receptors in the dorsal horn of the spinal cord, blocking substance P release and thereby blocking pain transmission.Epidural narcotics have a prolonged effect because their excretion is dependent on diffusion from CSF into the blood which is slow. Fentanyl is lipid soluble and has a duration of action of six hours. Morphine is more water soluble and can last up to 24 hours, so requires more intense postoperative monitoring. Adding a local anaesthetic reduces the opioid dose and is more efficacious in the prevention of secondary muscle spasm and sympathetic hyperreactivity.
Nerve Blocks and Local Anaesthetic Infiltration
Local anaesthetic agents can be effectively used to block peripheral nerves carrying pain fibres from the operative site or for local infiltration of the wound or operative site for postoperative pain relief. Bupivicaine is a commonly used agent which provides prolonged postoperative analgesia and is especially useful in the setting of day-care surgery where it facilitates early postoperative discharge.
INTRAVENOUS DRUG ADMINISTRATION
Prevention of Endocarditis
Antibiotic prophylaxis is essential to minimise the risk of endocarditis in patients for surgery who have:
- a heart valve lesion
- a septal defect
- patent ductus
- prosthetic valve
Standard regimes are detailed in the table below.
| Indication | Prophylaxis |
|---|---|
| Majority of at risk patients | Amoxicillin 1 g IV at induction Amoxicillin 500 mg p.o at six hours |
| Patients with prosthetic valve, or patients who have had previous infection | Amoxicillin 1 g plus gentamicin 120 mg IV at induction Amoxicillin 500 mg p.o. at 6 hours |
| Patients with allergy to penicillin | Vancomycin 1 g IV infusion pre-induction, plus gentamicin 120 mg IV at induction Teicoplanin 400 mg IV plus gentamicin 120 mg IV at induction Clindamycin 300 mg IV infusion pre-induction and 150 mg p.o or IV at 6 hours |
Aminophylline
Aminophylline is presented as 250 mg in a 10 ml ampoule and can be diluted in saline. A loading dose (5 mg/kg) should be given over the first 30 to 60 minutes and thereafter a maintenance infusion to maintain therapeutic plasma levels.The standard infusion rate is 0.5 mg per kilogram per hour and the standard infused solution is usually 500 mg in 500 ml, giving a concentration of 1mg/ml. Cardiac monitoring is important.
Hydrocortisone
This can be administered by slow IV injection, 100 mg every six hours. It can also be administered by IV infusion diluted in saline. Perioperative intravenous steroids are commonly used and are an essential component of the perioperative care of patients on preoperative oral steroid therapy.Intravenous hydrocortisone 100 mg every 4-6 hours is commonly used in the first 24-48 postoperative hours and reduced subsequently. Oral steroids can be resumed once gastrointestinal tract function has recovered.
Adrenaline, Noradrenaline
A common infusion schedule is 3 mg in 50 ml of dextrose 5%, where 1 ml per hour means delivery of 1 mcg per minute. The usual adult dose is 1-12 ml/hr.
Dobutamine
Dobutamine is normally made up as 250 mg in a 50 ml syringe of NaCl and infused at a preset rate based on patient weight and using a set table. Dobutamine dosage schedule based on a solution of 250 mg dobutamine in 50 ml of NaCl.
Dopamine
Dopamine is normally made up as 200 mg in 50 ml syringe and, in the same manner as dobutamine infused at a preset rate based on patient weight and using a set table. The dopamine dosage schedule is based on a solution of 200 mg dopamine in 50 ml of NaCl.
Heparin
The normal loading dose for a heparin infusion is 5000 international units IV over 5 minutes. It is normally titrated to an activated partial thromboplastin time ratio (aPTTR) of 1.5 to 2.5. The aPTTR should be measured six hours after the loading dose, 10 hours after a dose alteration and every four hours if the aPTTR is greater than five and daily once the therapeutic range has been reached. Guidelines exist for altering infusion rate IV depending on the result of the aPTTR.
Heparin Infusion Rate
| APTT ratio | Recommended Infusion Rate Change |
|---|---|
| 7 | Stop for 30 minutes to one hour and reduce by 500 units per hour |
| 5-7 | Reduce by 500 units per hour |
| 4-5 | Reduce by 300 units per hour |
| 3-4 | Reduce by 100 units per hour |
| 2.6-3 | Reduce by 50 units per hour |
| 1.5-2.5 | No change |
| 1.2-1.4 | Increase by 200 units per hour |
| 1.2 | Increase by 400 units per hour |
Metabolic and Nutritional Support
Introduction
Care of the surgical patient requires a thorough understanding of the fluid, electrolyte and nutritional changes that frequently accompany surgical disease. Surgical patients are especially prone to such disturbances because of:
- sepsis
- third space losses
- gastrointestinal disorders
- prolonged fasting
Prevention and correction of fluid, electrolyte and nutritional deficiencies are fundamental requirements for patient recovery. Your aim in managing fluid and electrolyte balance is to support normal homeostasis. This requires a knowledge of normal regulatory mechanisms, and the changes that occur in response to disease and operative trauma.
Because of the restriction of perioperative oral fluid intake and the administration of intravenous fluid and electrolyte replacement, regulation of intake is under your direct control. Management of fluid and electrolyte shifts in the surgical patient needs to be directed by repeated clinical and biochemical monitoring.
Between 30% and 60% of hospitalised surgical patients suffer from a degree of malnutrition as a result of inadequate caloric intake or as a result of the body's metabolic response to disease or stress. Malnutrition can impair patient recovery through its effects on mental status, muscle function and immunity so its prevention and correction is important. Surgeons need to know:
- how to assess nutritional status and requirements
- how best to deliver nutrition in different clinical situations
- how to manage the complications associated with different methods of nutritional delivery
Topic 1 – Fluid & Electrolyte Management
Normal Fluid
In neonates, water accounts for 75-80% of body weight. By age five, this decreases to 60-70% and continues to decrease throughout life remaining higher in males than females, and in lean as compared to obese people.
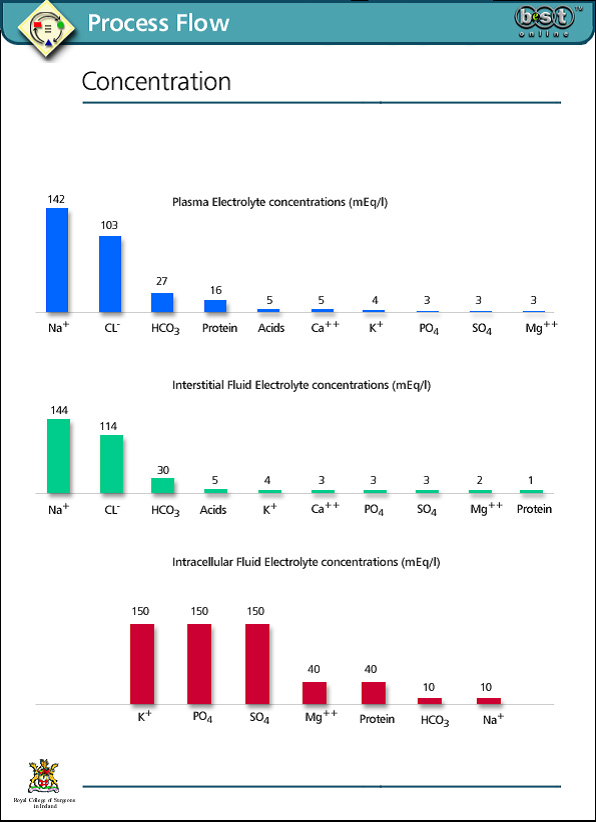
Two thirds of total body water is intracellular (ICF). One third is extracellular (ECF), the extracellular fluid compartment being divided into interstitial fluid and intravascular fluid (plasma). The intracellular and extracellular spaces in a 70 kg man would comprise 60% of total body weight: 40% intracellular, and 20% extracellular, divided between interstitial fluid at 15% and plasma at 5%.
Electrolyte Homeostasis
Plasma osmolality is normally between 275-300 mOsm/l. Although plasma proteins account for a relatively small proportion of the osmotically active particles in the intravascular space, they constitute one of the major differences between intravascular fluid and interstitial fluid. They are therefore of great importance in maintaining separation of intravascular and interstitial fluid. Urea and glucose make a relatively small contribution to osmolality normally, but may be significant in some disease states such as diabetic ketoacidosis.
Fluid and electrolyte balance is maintained by a complex interaction of central and peripheral neurohumoral mechanisms involving interplay between peripheral and central volume receptors, osmoreceptors and chemoreceptors and the hypothalamic-pituitary axis, the renin-aldosterone system and the kidneys.
Normal Maintenance Requirements
Fluid and electrolyte requirement calculations are based on body weight. Fluid loss occurs in the urine, faeces, and by insensible loss, principally through evaporation from the skin and bronchial tree.
Normal levels are as follows:
- the normal daily intake of water for a 70 kg man is approximately 2000-2500 ml
- normal requirement for sodium is 1 mmol/kg/day and for potassium is 0.5 mmol/kg/day
- a minimum of 600-800 ml/day of urine is needed to excrete the solutes produced by catabolic processes
- fluid loss in faeces is normally between 250-400 ml/day
- insensible loss is approximately 600 ml/day and occurs from the lungs by loss of moisture in expired air and from the skin by sweating
Insensible losses increase as a result of pyrexia, hyperventilation and hypermetabolic states such as hyperthyroidism. Evaporation from the open peritoneal cavity during prolonged abdominal surgery contributes substantially to insensible losses.
Holliday-Segar
Estimation of 24 hour fluid requirement (Holliday-Segar method)
| For Each Kilo | Add to Hourly Requirement |
|---|---|
| 0-10 kg | 4 ml/kg |
| 11-20 kg | 2 ml/kg |
| > 20 kg | 1 ml/kg |
For example: A 50 kg patient needs (10 x 4) + (10 x 2) + (30 x 1) = 90 ml/hour
Estimation of Pre-existing Deficit
Clinical signs of dehydration include tissue fluid loss and cardiovascular compromise. When the volume loss is acute, cardiovascular signs tend to predominate and when fluid depletion has been gradual, signs of tissue fluid loss are more marked. Clinical and biochemical evidence of dehydration include:
- dry mucous membranes
- reduced skin turgor
- tachycardia
- hypotension
- altered consciousness
- elevated haematocrit
- raised urea combined with normal creatinine
- hypernatraemia and hyperkalaemia
- increased plasma osmolality
Types of Intravenous Fluid
Crystalloid Solutions
Crystalloid solutions are composed of water with electrolytes and/or dextrose. Dextrose provides extra osmotic load so that these solutions are isotonic with plasma, but there is not enough to contribute significantly to nutritional requirements. The electrolyte components equilibrate rapidly with the extravascular space so the effect of these solutions on plasma volume is transitory.
Colloid Solutions
Colloid solutions contain macromolecules that are retained in the plasma for longer. Commonly used solutions are based on human albumin, dextrans, gelatin or starch molecules. These have a more sustained effect on intravascular volume and may be used for volume replacement. Their effectiveness depends on an intact vascular capillary basement membrane. If it is leaky, such as in severe burns or sepsis, oncotically active particles and fluid pass out of the intravascular space, leading to increased extravascular fluid loss.

Electrolytes
Constitution in mmol/l of commonly used electrolyte solutions.
| Solution | Na | K | Cl | Ca | Lactate | Dextrose | Calories | Osmolarity |
|---|---|---|---|---|---|---|---|---|
| Normal Saline | 154 | 0 | 154 | 0 | 0 | 0 | 0 | 292 |
| Solution | 31 | 0 | 31 | 0 | 0 | 40 | 160 | 0 |
| Hartmann's solution | 130 | 4 | 109 | 3 | 28 | 0 | 0 | 0 |
| 5% Dextrose | 0 | 0 | 0 | 0 | 0 | 50 | 200 | 274 |
Designing a Fluid Regime
Different combinations of electrolyte solutions can be given to supply 24-hour maintenance requirements.
Following correction of a deficit, the required fluid volume can be estimated by adding maintenance requirements to ongoing losses. Maintenance fluids need to provide sufficient free water and some, but not too much, sodium: 0.18% sodium chloride and 4% dextrose solution (solution 18) is commonly used.
The choice of replacement fluid to cover losses depends on the composition of the body fluid that is being lost. Normal saline with added potassium is used to replace gastric losses, but for most gastrointestinal losses Hartmann's solution is the most physiologic and suitable.
Intraoperative Fluid Therapy
Intraoperative fluid therapy requires correction for the ECF volume that is lost by evaporation from the open peritoneal surfaces and by tissue sequestration at the operative site. The amount lost depends on the magnitude of the operative trauma and the duration of the operation and should be replaced with a crystalloid solution. Blood loss is replaced with crystalloid initially, colloid with increasing blood loss and with blood if it exceeds more than 10-15% of blood volume.

Surgical Stress
Fluid requirement during the first two postoperative days is affected by the physiological response to surgical stress. Increased production of ADH, aldosterone and glucocorticoid hormones result in increased retention of fluid, sodium and potassium. Baseline fluid requirement during this period is reduced to approximately 2 litres/24 hours for a 70 kg male, without the need for extra potassium. However, such calculations are frequently complicated by a persistent deficit because of incomplete replacement of intraoperative losses, and by continuing losses depending on the procedure performed.
Frequent Review is Crucial
Postoperative fluids need to be reviewed frequently with regular evaluation of the patient. Reliance on rigid formulas to calculate fluid requirements is not good practice. The early postoperative period — when fluid retention and hypoalbuminaemia may be present — is a high-risk time for cardiac decompensation due to iatrogenic fluid overload.
Abnormalities of Fluid and Electrolyte Status
In general, abnormalities of fluid and electrolyte status may be due to changes in volume, concentration or composition. They rarely occur in isolation from one another.
Changes in Volume
Loss of volume is one of the most common fluid abnormalities in surgical patients. When loss of volume is due to acute bleeding, the immediate priority is replacement of volume. Initially, 2L of Hartmann's solution is recommended and, although up to 75% of this will be redistributed rapidly outside the intravascular space, its composition is close to that of ECF so it will not result in any acute electrolyte imbalance. If signs of hypovolaemia persist or worsen, it is likely that a moderate to severe bleed has occurred, so cross-matched blood should be given.
External Loss
Volume loss unrelated to bleeding occurs when there is excess external loss or third space loss. Most external loss occurs from the extracellular space. Provided the fluid lost is isotonic, accompanying shifting and reduction of intracellular fluid volume occurs gradually. Changes of ECF osmolality affect ICF volume more rapidly. If there is no evidence of haemodynamic instability, volume replacement may proceed more slowly. The rate of replacement may have to be reduced in patients with limited cardiorespiratory function and monitored by measuring CVP.
Corrected Quickly, Returns to Normal
Volume loss leads to elevation of serum urea and creatinine (prerenal uraemia). If corrected promptly, renal function returns to normal. If volume depletion and hypotension are not corrected, acute tubular necrosis and intrinsic renal uraemia may result.
Distinction between pre-renal uraemia and acute tubular necrosis or intrinsic renal failure can be difficult in practice. Traditional diagnostic indices as tabulated in the clinical note may not be reliable after intravenous fluids or diuretics have been given. It is reasonable to treat pre-renal failure with intravenous fluid challenges until repeated clinical assessment shows that there is no longer a volume deficit. Once renal failure has become established, fluids may be restricted to match ongoing losses. Consideration should be made about renal replacement therapy.
Changes in Concentration and Composition
Abnormalities of electrolyte concentration and composition are best considered individually though they rarely occur in isolation from each other. Abnormally low or high levels of any plasma electrolyte can occur and each abnormality is associated with specific aetiological factors and clinical features and requires a thoughtful approach to management.
Abnormalities of sodium and potassium are common in surgical practice. Changes in calcium, magnesium and phosphate occur less frequently but may have important implications.
Abnormalities of Acid Base Balance
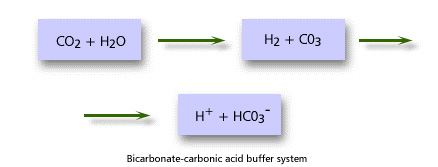
The pH of body fluids must be tightly regulated to maintain molecular structure and enzyme function. A variety of mechanisms known as buffer systems operate to keep extracellular fluid at a pH between 7.35 and 7.45. Intracellular pH is difficult to measure but is more acidic than the ECF.
Buffer systems are able to resist changes in pH when small amounts of acid or alkali are added. They are able to take up and release protons quickly so that the number of free protons and hence pH remain within a narrow range. The most important buffer system in plasma is the bicarbonate-carbonic acid system. Haemoglobin and plasma proteins also contribute some buffer function.
Ultimately control of acid-base balance depends on the kidneys and the lungs. Changes in ventilation allow adjustment in CO2 excretion that can directly affect acid-base balance. The kidneys adjust acid-base balance by either retention/excretion of bicarbonate and excretion/retention of free hydrogen ions.
Acid-base balance is estimated by measurement of arterial blood gases. Disorders are divided into:
- respiratory acidosis
- respiratory alkalosis
- metabolic acidosis
- metabolic alkalosis
Interpretation of the blood gas values is made more difficult by the presence of superimposed changes produced by the physiological compensatory mechanisms. The blood gas findings and causes of these different acid-base abnormalities are shown in the table:
| Acid-Base Abnormaility | Blood Gas Findings | Compensatory Responses | Causes |
|---|---|---|---|
| Respiratory Acidosis | Decrease pH increase pC02 | Increase HC03 | Ventilatory Failure |
| Resouratiry Alkalosis | Increase pH decrease pC02 | Decrease HC03 | Hyperventilation, Hypoxia, excess mechanical ventilation, central causes |
| Metabolic Acidosis | Decrease pH decrease HC03 | Decrease pC02 | Sepsis, renal impairment, diabetic ketoacidosis, excess GI or renal HC03 loss |
| Metabolic alkalosis | Increase pH increase HC03 | Increase pC02 | Excess acid loss from stomach or kidney hyperaldosteronism |
Topic 2 – Nutrition in the Surgical Patient
Malnutrition in Surgical Patients
Malnutrition commonly accompanies a variety of diseases. Many gastrointestinal diseases, for example, can interfere with absorption of nutrients from the intestine making it difficult to maintain adequate caloric intake.
Cancer is commonly associated with profound malnourishment that is multifactorial in origin. While management of these conditions should include correction of the nutritional state, it is worrying that hospitalisation generally causes a worsening of the nutritional state.
Adverse Consequences of Malnutrition
It has been shown that hypoalbuminaemia or loss of more than 10% of body weight increase the risk of hospital morbidity and mortality, and lengthen hospital stay. Malnutrition contributes to decreased cell-mediated immunity, increased risk of sepsis and poor wound healing.
Clinical Types of Malnutrition
Marasmus
This is due to a deficiency of all dietary components: protein intake and total caloric intake are inadequate. Somatic muscle protein is used as an energy source resulting in wasting and emaciation with little or no peripheral oedema. In hospital, this is seen in patients with, for example obstructing upper gastrointestinal tumours or anorexia nervosa.
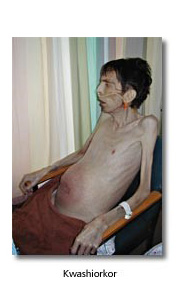
Kwashiorkor
Here protein intake is reduced but total caloric intake is not. Visceral protein is broken down while somatic protein and subcutaneous fat are spared. Anthropometric measurements may be misleading, and oedema may further confuse the issue.
Hypoalbuminaemic Malnutrition
Marasmus and kwashiorkor rarely exist in pure form. In hospital, the picture is usually altered by the effects of the physiological response to stress and disease. This results in a catabolic process that profoundly affects the metabolic utilisation of food. In addition to inadequate intake, malnutrition occurs because of the inability to use ingested foodstuffs. The fall in serum albumin seen in these stressed situations is far more precipitous than would be seen in simple starvation.
Overnutrition
Obesity is another form of malnutrition. However, this is included here for the sake of completeness only — the remainder of this topic deals solely with the problem of undernutrition
Calculating Nutritional Requirements
Calculation of nutritional requirements should consider the patient's need for calories, nitrogen/protein and other nutrients. Caloric and protein requirements are dependent on the degree of physiological stress and the metabolic state.
Calories
Caloric needs are influenced by:
- basal metabolic rate (BMR)
- levels of activity
- stress factor — extra energy required because of physiological stress
Theoretical caloric needs may be calculated as the BMR with corrections to account for the degree of physical activity and the degree of physiological stress such as injury or inflammation.
Pragmatically, daily caloric requirements are usually met by 25-30 kcal/kg body mass. Half is given as carbohydrates, half as fats.
Septic patients are hypercatabolic and have greater energy expenditure. However, matching this by intake causes hyperglycaemia and liver dysfunction.
Protein
Protein intake is usually expressed as g Nitrogen (6.25 g protein = 1 g nitrogen).
Initial daily protein intake should be 1 – 1.2 g/kg, or 0.15-0.2 g/kg N2.
While septic patients may lose protein equivalents > 0.3 g/kg N2, it is not possible to match this by intake. The emphasis of management is to control the source of sepsis.
Other Nutrients
Proper nutrition includes several other dietary elements including vitamins, minerals and trace elements. A mixed formula enteric feed usually contains sufficient quantities of these components. However, in some disease states extra amounts must be added, or parenteral administration must be considered. An example would be the need for vitamin K in patients with obstructive jaundice.
Stress Factor
Calculation of Stress Factor
| Physiological Stress | Percentage Change in Basal Metabolic Rate |
|---|---|
| Starvation | 0 - 20% |
| Post-op, Mild sepsis | 0 - 13% |
| Major fracture, Moderate sepsis | 7 - 25% |
| Polytrauma, Severe sepsis | 26 - 50% |
| Severe sepsis & Ventilation | 40 - 70% |
| Full thickness burn 10% | 20% |
| Full thickness burn 20% | 40% |
| Full thickness burn 30% |
Delivering Nutritional Support
Nutritional support may be delivered by either:
- the gastrointestinal tract — enteral feeding
- the intravenous route — total parenteral feeding (TPN)
Enteral feeding has many advantages over total parenteral feeding and should generally be used unless there is a gastrointestinal contraindication such as
- ileus
- mechanical bowel obstruction
- severe diarrhoea
In circumstances where nutrition cannot be entirely enteral, it is worth giving a small amount this way to:
- prevent mucosal atrophy
- maintain mucosal immunological function
The effectiveness of nutritional support should be monitored by repeated clinical and laboratory evaluation.
